Unleash Power Within: Finite Element Modeling of Temporal Interference Stimulation of Phrenic Nerves
Introduction: Mechanical ventilation in the ICU can result in complications, including ventilator-induced diaphragmatic dysfunction (VIDD), leading to extended hospital stays and increased costs. This abstract presents a novel approach using multiple esophageal electrodes for phrenic nerve stimulation (PNS) to address VIDD. Finite element modeling enhances our understanding and optimization of stimulation techniques. Esophageal electrodes offer selective stimulation and diaphragmatic electromyography monitoring advantages. Temporal interference stimulation (TIS) shows promise in overcoming limitations of conventional techniques. This study assesses the feasibility of efficient PNS for diaphragm activation while minimizing co-stimulation. Using a comprehensive finite element model developed with COMSOL Multiphysics®, crucial parameters such as electrode size, distance, and positioning are systematically varied to identify the optimal configuration. The research aims to enhance the understanding of electrical field distribution in TIS through finite element analysis and enable an optimized configuration for precise deep stimulation. Simulation results inform the design of an effective stimulation protocol.
Methods: This study investigated three electrode placement approaches: internal (esophageal), external (transcutaneous cervical), and mixed (esophageal and transcutaneous dipole). Using the COMSOL Multiphysics® software and the AC/DC Module, their effects on stimulation outcomes were evaluated. Control over interference site was achieved through current amplitude ratios and electrode placement. Stimulation frequency, waveform, and electrode design were analyzed using a complex system. The external approach positioned dipole electrodes on the skin surface surrounding the neck, see figure 1. In the internal approach, dipole electrodes were placed esophageally using a custom-developed catheter. The mixed approach combined external and internal placement. The electrode-skin interface considered skin as a multilayer structure with specific electrical properties. Variations in electrode position required accounting for additional geometric and physiological properties. A time-dependent study with a parametric sweep was conducted, and MATLAB® was used for post-processing tasks.
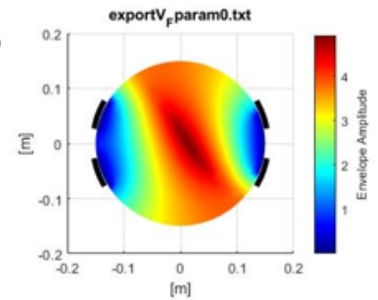
Results The results indicate that precise electrode placement plays a crucial role in steering the external envelope locus during TIS, figure 2 and 3. The envelope locus, generated in post-processing, represents the maximum stimulation depth for each parameter setting. Varying the locations of electrodes has a significant impact on control capabilities, whereas variations in electrode size have minimal effect. The study introduces the potential of controlling the envelope locus without physically moving the electrodes, presenting non-invasive control possibilities. By optimizing stimulation amplitudes, smaller and more targeted regions can be activated, enhancing precision in deep neural stimulation.
Discussion The findings of this study emphasize the significance of accurate electrode positioning in regulating the external envelope location during TIS, aligning with existing literature [3]. Nevertheless, it is crucial to note that the current model is a simplified representation and additional enhancements are necessary to more accurately reflect real-world physical conditions. Future investigations should prioritize optimizing the model by integrating more realistic anatomical structures and electrical properties and validate the results through in vitro verification and in vivo validation. Factors such as patient-specific variations should be considered to enhance the effectiveness and safety of TIS in clinical settings.
Téléchargement
- Kaufmann_6501_poster.pdf - 0.73MB
