La Bibliothèque d'Applications présente des modèles construits avec COMSOL Multiphysics pour la simulation d'une grande variété d'applications, dans les domaines de l'électromagnétisme, de la mécanique des solides, de la mécanique des fluides et de la chimie. Vous pouvez télécharger ces modèles résolus avec leur documentation détaillée, comprenant les instructions de construction pas-à-pas, et vous en servir comme point de départ de votre travail de simulation. Utilisez l'outil de recherche rapide pour trouver les modèles et applications correspondant à votre domaine d'intérêt. Notez que de nombreux exemples présentés ici sont également accessibles via la Bibliothèques d'Applications intégrée au logiciel COMSOL Multiphysics® et disponible à partir du menu Fichier.
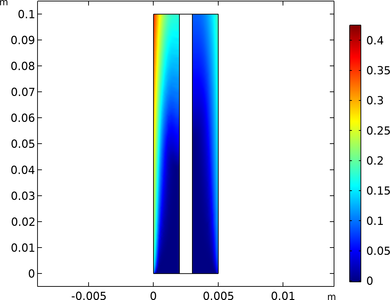
This example applies the Electrophoretic Transport and Laminar Flow interfaces to model isoelectric separation in a free-flow electrophoresis device. A stream containing six different ionic species is shown to be divided into pure component streams by means of migrative transport in an ... En savoir plus
Deposition of metallic lithium on the negative electrode in preference to lithium intercalation is known to be a capacity loss and safety concern for lithium-ion batteries. Harsh charge conditions such as high currents (fast charging) and/or low temperatures can lead to lithium plating. ... En savoir plus
In the diffuse double layer and within the first few nanometers of an electrode surface, the assumption of electroneutrality is not valid due to charge separation. Typically, the diffuse double layer may be of interest when modeling very thin layers of electrolyte including those in ... En savoir plus
The copper current collector on negative graphite electrodes in lithium-ion batteries have been seen to dissolve at over discharge. This can be a safety concern as the dissolution damages the current collector irreversibly and dissolved copper ions can redeposit and form dendrites. ... En savoir plus
Alkaline water electrolysis is a well-established industrial process for producing hydrogen gas. In the cell, hydrogen gas is formed at the cathode whereas oxygen gas is formed at the anode. The electrolyte is an aqueous liquid, and when the evolved gases form bubbles, the effective ... En savoir plus
This model exemplifies how to compute the internal temperature distribution in a prismatic battery during a high-rate charge. The electrochemistry is described by a a lumped two-electrode model, which is coupled to the heat transfer model. The heat transfer model includes the effects of ... En savoir plus
The electrochemical cell shown in this model can be regarded as a unit cell of a larger wire-mesh electrode that is common in many industrial processes. One of the most important aspects in the design of electrochemical cells is the current density distributions in the electrolyte and ... En savoir plus
Electrochemical supercapacitors feature relatively higher energy densities than conventional capacitors. With several advantages, such as fast charging, long charge–discharge cycles, and broad operating temperature ranges, electrochemical supercapacitors have found wide applications in ... En savoir plus
This model defines a zero-gap alkaline water electrolyzer, where oxygen and hydrogen gas are evolved in porous gas diffusion nickel felt electrodes, placed adjacent to a porous separator (diaphragm). The geometry defines a unit cell of an electrolyzer stack, in turn comprising two full ... En savoir plus
Some positive electrode materials are known to deteriorate in overcharged lithium-ion battery cells. Predominantly, manganese containing electrode materials such as LMO and NMC can loose capacity due to manganese dissolving from the materials at overcharge. This decomposition is a ... En savoir plus