La Bibliothèque d'Applications présente des modèles construits avec COMSOL Multiphysics pour la simulation d'une grande variété d'applications, dans les domaines de l'électromagnétisme, de la mécanique des solides, de la mécanique des fluides et de la chimie. Vous pouvez télécharger ces modèles résolus avec leur documentation détaillée, comprenant les instructions de construction pas-à-pas, et vous en servir comme point de départ de votre travail de simulation. Utilisez l'outil de recherche rapide pour trouver les modèles et applications correspondant à votre domaine d'intérêt. Notez que de nombreux exemples présentés ici sont également accessibles via la Bibliothèques d'Applications intégrée au logiciel COMSOL Multiphysics® et disponible à partir du menu Fichier.
Steel pipelines are often subjected to complex stress/strain conditions in the oil and gas industry. Additionally, pipes are subjected to significant longitudinal strain due to soil movement. For the elastoplastic stress simulation, the Solid Mechanics interface is used with the small ... En savoir plus
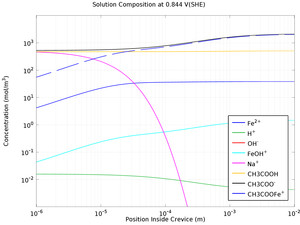
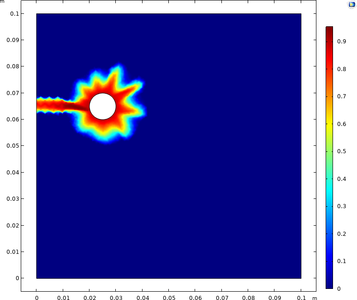
Mass transport limitations within thin crevices can often result in the local electrochemistry to differ significantly between the crevice opening (mouth) and end (tip), and as a result of the differences in local chemistry, corrosion may occur. This example models crevice corrosion of ... En savoir plus
This example models galvanic corrosion between two different phases in a magnesium alloy for a representative cross-sectional microstructure configuration. The Phase Field interface is used here to model dissolution of a constituent phase leading to topological changes. The electrode ... En savoir plus
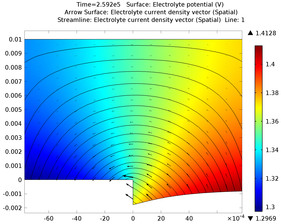
Voltammetry is modeled at a microelectrode of 10um radius. In this common analytical electrochemistry technique, the potential at a working electrode is swept up and down and the current is recorded. The current-voltage waveform ("voltammogram") gives information about the reactivity and ... En savoir plus
This tutorial example models the currents and the concentration of dissolved metal ions in a battery (corrosion cell) made from an orange and two metal nails. This type of battery is commonly used in chemistry lessons. Instead of an orange, lemons or potatoes can also be used. En savoir plus
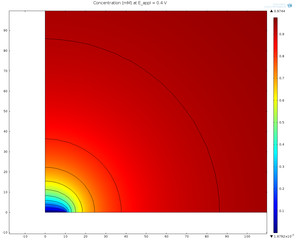
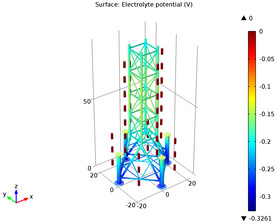
Steel structures immersed in seawater can be protected from corrosion through cathodic protection. This protection can be achieved by an impressed external current or by using sacrificial anodes. The use of sacrificial anodes is often preferred due to its simplicity. This example models ... En savoir plus
This model simulates atmospheric galvanic corrosion of a busbar, which includes a copper flange, an aluminum alloy flange in contact with a zinc nut and bolt. The Secondary Current Distribution interface is used to solve for the electric potential in electrode domain and Current ... En savoir plus
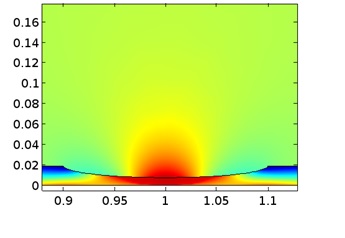
This 2D model demonstrates how to model a galvanic couple in which the corrosion of the anode causes a geometry deformation. The parameter data used is for an Magnesium Alloy (AE44) - mild steel couple in brine solution (salt water). En savoir plus
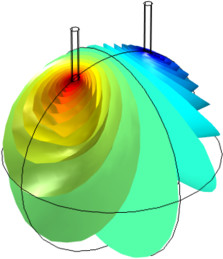
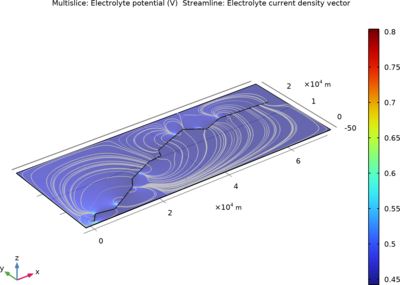
In this example, three parallel pipelines of length 68 km and a horizontal separation distance of 10 m between them are protected against corrosion by an impressed current cathodic protection (ICCP) system using a series of anodes. Each anode is connected to all three pipelines, ... En savoir plus
Oxide jacking is the process by which reinforced concrete cracks, due to the corrosion of the reinforcing rebar rods. The corrosion process causes growth of an oxide layer on the rebar, which in turn causes internal stresses in the concrete. If the corrosion process is allowed to ... En savoir plus