La Bibliothèque d'Applications présente des modèles construits avec COMSOL Multiphysics pour la simulation d'une grande variété d'applications, dans les domaines de l'électromagnétisme, de la mécanique des solides, de la mécanique des fluides et de la chimie. Vous pouvez télécharger ces modèles résolus avec leur documentation détaillée, comprenant les instructions de construction pas-à-pas, et vous en servir comme point de départ de votre travail de simulation. Utilisez l'outil de recherche rapide pour trouver les modèles et applications correspondant à votre domaine d'intérêt. Notez que de nombreux exemples présentés ici sont également accessibles via la Bibliothèques d'Applications intégrée au logiciel COMSOL Multiphysics® et disponible à partir du menu Fichier.
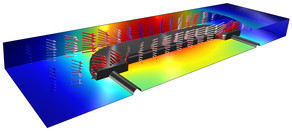
The model simulates non-premixed turbulent combustion of syngas (synthesis gas) in a simple round-jet burner. Syngas is a gas mixture, primarily composed of hydrogen, carbon monoxide and carbon dioxide. The name syngas relates to its use in creating synthetic natural gas. In the model, ... En savoir plus
This tutorial illustrates how to use the Uncertainty Quantification (UQ) functionality to answer questions regarding sensitivity and reliability of a flow reactor with thermal decomposition. The tutorial investigates what uncertainties in parameters dominates the survival of a nutrient ... En savoir plus
This 2D example of a vanadium flow battery demonstrates how to couple a secondary current distribution model for an ion-exchange membrane to tertiary current distribution models for two different free electrolyte compartments of a flow battery. The Ion-Exchange Membrane boundary node ... En savoir plus
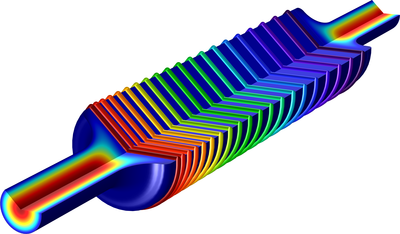
Tutorial model of electroplating. The model uses secondary current distribution with full Butler-Volmer kinetics for both anode and cathode. The thickness of the deposited layer at the cathode is computed as well as the pattern caused by dissolution of the anode surface. En savoir plus
This tutorial models the cathodic protection of an oil rig structure during a time period of 15 years. As a result of the consumption of the sacrificial anodes, the protective capabilities system are reduced over time. The anode shape change in the model is defined by using the Level ... En savoir plus
One of the most common reactors in the chemical industry, for use in heterogeneous catalytic processes, is the packed bed reactor. This type of reactor is used both in synthesis as well as in effluent treatment and catalytic combustion. This model is set up to calculate the ... En savoir plus
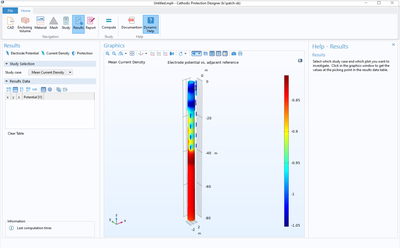
The Cathodic Protection Designer application is an example of how an application can be used to simplify the simulation process by featuring a way to import a generic CAD file with certain requirements. Using this app, the user can select each part of the geometry and set boundary ... En savoir plus
Carbon deposition on the surface of solid catalysts is commonly observed in hydrocarbon processing. A known problem is that carbon deposits can impede the activity of catalysts as well as block the flow of gas through a catalyst bed. This example investigates the thermal decomposition ... En savoir plus
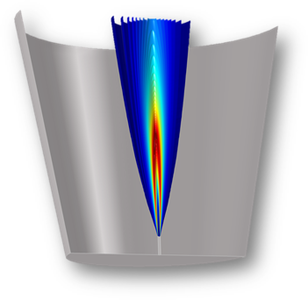
Pitting corrosion is a type of localized corrosion by which local cavities, pits, are formed on an initially smooth metal surface. A pit may be initialized by surface defects, such as an inhomogeneities in composition or shape, or mechanical abuse resulting in a small scratch or dent. ... En savoir plus
In a lithium metal battery, lithium metal is deposited during charging on the negative electrode. Mass transport and ohmic effects in the electrolyte cause small protrusions on the metal surface to be subjected to accelerated growth during charging. In worst case scenarios, this leads to ... En savoir plus