La Bibliothèque d'Applications présente des modèles construits avec COMSOL Multiphysics pour la simulation d'une grande variété d'applications, dans les domaines de l'électromagnétisme, de la mécanique des solides, de la mécanique des fluides et de la chimie. Vous pouvez télécharger ces modèles résolus avec leur documentation détaillée, comprenant les instructions de construction pas-à-pas, et vous en servir comme point de départ de votre travail de simulation. Utilisez l'outil de recherche rapide pour trouver les modèles et applications correspondant à votre domaine d'intérêt. Notez que de nombreux exemples présentés ici sont également accessibles via la Bibliothèques d'Applications intégrée au logiciel COMSOL Multiphysics® et disponible à partir du menu Fichier.
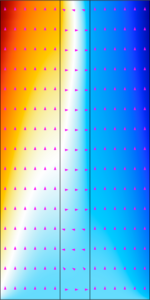
This tutorial demonstrates how to modify a concentration-independent (also known as a secondary current distribution) liquid alkaline water electrolyzer with concentrated electrolyte theory to explicitly resolve local electrolyte and solvent concentrations (thereby creating a tertiary ... En savoir plus
This model defines a zero-gap alkaline water electrolyzer, where oxygen and hydrogen gas are evolved in porous gas diffusion nickel felt electrodes, placed adjacent to a porous separator (diaphragm). The geometry defines a unit cell of an electrolyzer stack, in turn comprising two full ... En savoir plus
In an alkaline electrolyzer stack, all cells share the same electrolyte. As a result of all cells being in ionic contact, parasitic shunt currents flow between the cells through the manifolds and the electrolyte channels, on both the inlet and outlet side. This example models a ... En savoir plus
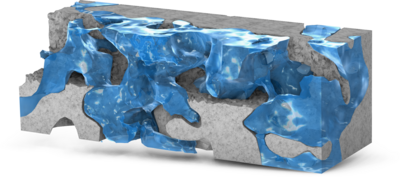
Digital Rock technology utilizes high-resolution imaging techniques, such as SEM and X-ray CT, to characterize the pore structures of rock cores. This approach enables pore-scale modeling of reservoir rocks, offering valuable insights into pore morphology, connectivity, and fluid ... En savoir plus
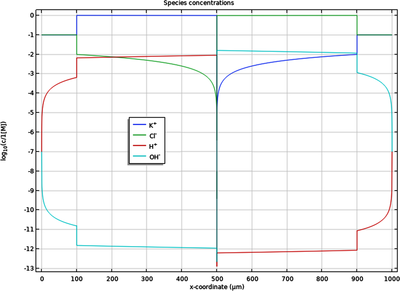
The chlor-alkali membrane process is one of the largest in industrial electrolysis with the production of roughly 40 million metric tons of both chlorine and caustic soda per year. Chlorine is used predominantly for the production of vinyl chloride monomer, which in turn is used for the ... En savoir plus
This example models co-electrolysis of H2O and CO2 using a solid oxide electrolyzer cell. The model includes the full coupling between the mass balances and gas flow in the H2 and O2 gas diffusion electrodes, the momentum balances in the H2 and O2 gas flow channels, the energy balance ... En savoir plus
Alkaline water electrolysis is a well-established industrial process for producing hydrogen gas. In the cell, hydrogen gas is formed at the cathode whereas oxygen gas is formed at the anode. The electrolyte is an aqueous liquid, and when the evolved gases form bubbles, the effective ... En savoir plus
This model file was used for creating the plots featured in the blog post "How to Model Ion-Exchange Membranes and Donnan Potentials". En savoir plus
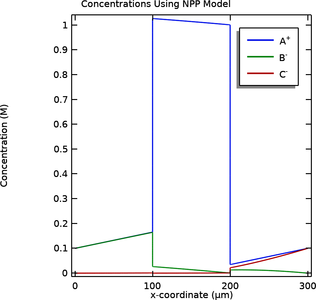
A bipolar membrane consists of one anion-selective, and one cation-selective membrane, in contact with each other. The combined cation and anion selectivity makes the bipolar membrane highly impermeable to all ions, with the exception of H+ and OH- which are formed by water splitting ... En savoir plus
Metal hydride tanks offer safe hydrogen storage, thanks to their low reactivity, and a relatively high hydrogen density. When developing metal hydride hydrogen tank designs, modeling and simulation is useful for optimizing operating conditions, such as gas composition, pressure, and ... En savoir plus