- Bridging the Terahertz Gap
- Modeling the Lithium-Ion Battery
- Protection contre la Corrosion
- Modélisation des batteries
- Modélisation et Simulation dans le développement des piles à combustible
- Modélisation thermique des petits satellites
- Analyse électro-vibroacoustique d'un transducteur à armature équilibrée
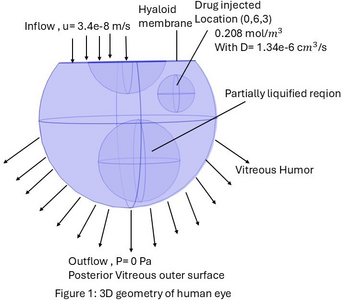
Computational Modeling of Drug Ranibizumab Transport in Age-Related Macular Degeneration Treatment (AMD)
Age-related macular degeneration (AMD) is an irreversible disease that causes vision loss, and nearly 11 million Americans are currently affected, with prevalence expected to double by 2050 due to an aging population. Vascular endothelial growth factor (VEGF) plays an important role in pathological angiogenesis by promoting the formation of vascular permeability and the growth of fragile, leaky blood vessels. Anti-VEGF therapies, such as ranibizumab, are commonly used as intravitreal injections in AMD treatment. This study develops a computational model to investigate the transport of ranibizumab within a partially liquified vitreous humor(VH), a common condition in aging eyes. We specifically analyze how three different injection locations affect drug distribution spatially and calculate the drug concentration in the vitreous humor. A three-dimensional (3D) model of the human eye(Figure 1), including the vitreous, retina, lens, and hyaloid membrane, was developed and solved using COMSOL Multiphysics 6.3. The geometry incorporated key ocular structures, including the retina, lens, and hyaloid membrane. Three distinct intravitreal injection locations were investigated: anterior (0,6,3 mm), middle (0,3,-0.5 mm), and posterior (0,0,-4 mm). The Darcy’s law is used to simulate fluid flow through the partially liquified VH, where both the liquified and the gel region are solved by the Darcy-Darcy equation with the permeability ratio KL/KG = 〖10〗^3. The transport of the drug ranibizumab was simulated by the “transport diluted species physics” using the convection diffusion-reaction equation. The diffusion coefficient of the drug ranibizumab for the human vitreous is fixed at D = 1.34×〖10〗^(-6) 〖cm〗^2/ s. No flux boundary condition was applied to the lens and the anterior vitreous interface, preventing any drugs and fluid from passing through it. The posterior region of the vitreous was defined as the primary outlet, representing the major route for drug elimination. A therapeutic dose of 0.5 mg/0.05 mL ranibizumab (0.2083[mol/m^3 ]) as an initial condition at each injection point. The simulation was run for 40 days to observe the drug concentration profiles. An "extra fine" physics-controlled mesh was utilized to ensure the numerical accuracy of the results. Our computational results demonstrate distinct spatial and temporal profiles of ranibizumab distribution depending on the injection site. Figure 2 includes a 2D drug transport plot highlighting how injection location influences intravitreal drug transport in liquefied vitreous conditions. Figure 3 includes the drug concentration plot in the human vitreous, comparing three different locations. This computational model successfully simulates the fluid flow and drug transport characteristics within the partially liquified vitreous humor. Our COMSOL model can provide impactful insights and a useful prediction of the drug delivery and optimal drug response in treating AMD disease.