La Bibliothèque d'Applications présente des modèles construits avec COMSOL Multiphysics pour la simulation d'une grande variété d'applications, dans les domaines de l'électromagnétisme, de la mécanique des solides, de la mécanique des fluides et de la chimie. Vous pouvez télécharger ces modèles résolus avec leur documentation détaillée, comprenant les instructions de construction pas-à-pas, et vous en servir comme point de départ de votre travail de simulation. Utilisez l'outil de recherche rapide pour trouver les modèles et applications correspondant à votre domaine d'intérêt. Notez que de nombreux exemples présentés ici sont également accessibles via la Bibliothèques d'Applications intégrée au logiciel COMSOL Multiphysics® et disponible à partir du menu Fichier.
The purpose of the app is to demonstrate and simulate the use of cyclic voltammetry. You can vary the bulk concentration of both species, transport properties, kinetic parameters, as well as the cycling voltage window and scan rate. Cyclic voltammetry is a common analytical technique ... En savoir plus
Copper electrowinning is the process of copper extraction from an electrolyte solution and its deposition at the cathode surface, by passing an external current through the electrolytic cell and using an insoluble anode. During the process, oxygen bubbles are generated at the anode ... En savoir plus
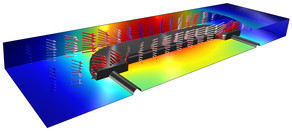
This example models electrocoating of paint onto a car door in a time-dependent simulation. The deposited paint is highly resistive which results in lowered local deposition rates for coated areas. A primary current distribution in combination with a film resistance model is used to ... En savoir plus
The purpose of this app is to understand EIS, Nyquist, and Bode plots. The app lets you vary the bulk concentration, diffusion coefficient, exchange current density, double layer capacitance, and the maximum and minimum frequency. Electrochemical impedance spectroscopy (EIS) is a common ... En savoir plus
Tutorial model of electroplating. The model uses secondary current distribution with full Butler-Volmer kinetics for both anode and cathode. The thickness of the deposited layer at the cathode is computed as well as the pattern caused by dissolution of the anode surface. En savoir plus
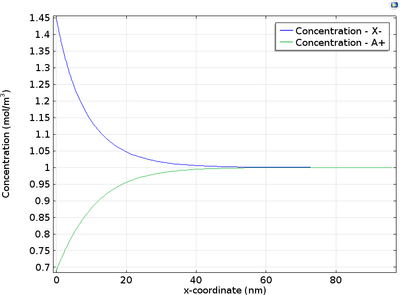
At the electrode-electrolyte interface, there is a thin layer of space charge in a diffuse double layer. This may be of interest when modeling devices such as electrochemical capacitors and nanoelectrodes. This tutorial example shows how to couple the Nernst-Planck equations to the ... En savoir plus
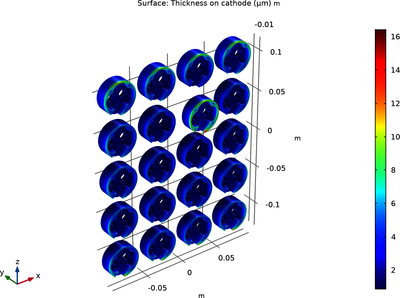
When several components are to be electroplated they are typically mounted on a rack in the electroplating bath. An important aspect is then achieving a uniform thickness of the plated layer for all components mounted on the rack. This example model allows for investigating the effect ... En savoir plus
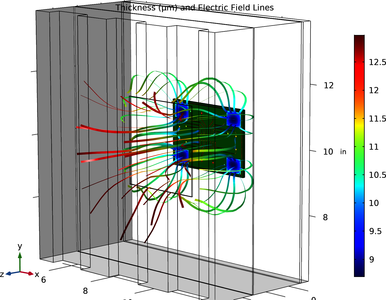
This example simulates electroplating of a printed circuit board (PCB) in 3D using the Secondary Current Distribution interface. In order to achieve thickness uniformity across the PCB, a dummy pattern is included in the design, along with an aperture in the electroplating bath. En savoir plus
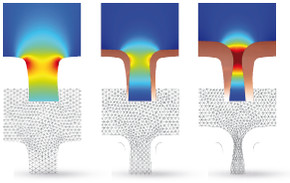
This model demonstrates the use of moving meshes in the application of copper electrodeposition on circuit boards. In these environments, the presence of cavities or 'trenches' are apparent. The model makes use of the Tertiary, Nernst-Planck interface for electrodeposition to keep track ... En savoir plus
This tutorial example models the currents and the concentration of dissolved metal ions in a battery (corrosion cell) made from an orange and two metal nails. This type of battery is commonly used in chemistry lessons. Instead of an orange, lemons or potatoes can also be used. En savoir plus