Solid Oxide Electrolyzer Using Thermodynamics
Application ID: 74001
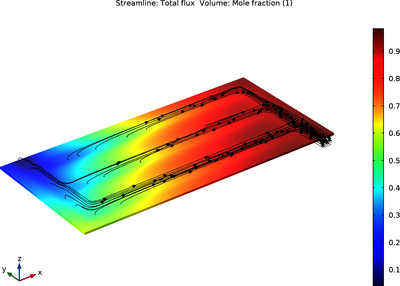
This example models a solid oxide electrolyzer cell wherein water vapor is reduced to form hydrogen gas on the cathode, and oxygen gas is evolved on the anode. The current distribution in the cell is coupled to the cathode mass transfer of hydrogen and water and momentum transport.
Two versions of the model file are provided. One version uses the Hydrogen Electrolyzer interface to define the electrochemistry, mass transfer, thermodynamics and mixture properties all within the same physics interface.
In the second version, separate physics interfaces are used to define the separate phenomena of the cell, and the Thermodynamics and Chemistry nodes are used to automatically define the properties of the cathode gas mixture, as well as the equilibrium potentials of the electrode reactions.
The second approach can be used as a template for setting up arbitrary electrolyzer cells, involving more complex chemistry.
This model example illustrates applications of this type that would nominally be built using the following products:
however, additional products may be required to completely define and model it. Furthermore, this example may also be defined and modeled using components from the following product combinations:
The combination of COMSOL® products required to model your application depends on several factors and may include boundary conditions, material properties, physics interfaces, and part libraries. Particular functionality may be common to several products. To determine the right combination of products for your modeling needs, review the Grille des Spécifications and make use of a free evaluation license. The COMSOL Sales and Support teams are available for answering any questions you may have regarding this.



