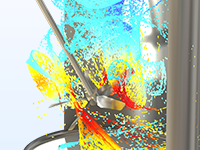
Produits chimiques de base
Simuler des mélangeurs à agitation continue dépendants de l'espace et des réacteurs à réservoirs pour des débits élevés.
Comprendre et optimiser les procédés chimique et leur conception
Les modèles mathématiques aident les scientifiques, les développeurs et les ingénieurs à comprendre les procédés, les phénomènes et les designs des systèmes chimiques réactifs. Le module Chemical Reaction Engineering, complémentaire de la plate-forme logicielle COMSOL Multiphysics®, fournit des interfaces utilisateur pour la création, l'inspection et l'édition d'équations chimiques, d'expressions cinétiques, de fonctions thermodynamiques et d'équations de transport. Après avoir développé un modèle validé, celui-ci peut être utilisé pour étudier différentes configurations et conditions de fonctionnement des systèmes réactifs et des phénomènes de transport associés. La résolution répétée des équations du modèle pour différentes entrées conduit à une véritable compréhension du système étudié.
Contacter COMSOL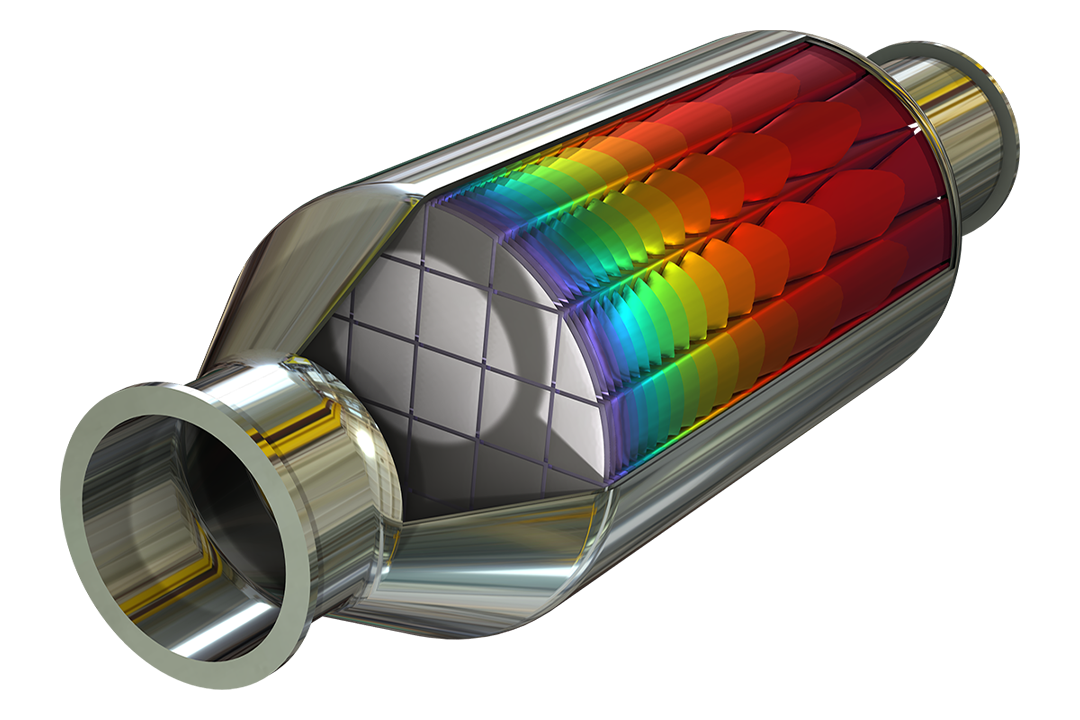
Modéliser les phénomènes de transport et les réactions chimiques dans de nombreux procédés industriels avec le logiciel COMSOL®.

Simuler des mélangeurs à agitation continue dépendants de l'espace et des réacteurs à réservoirs pour des débits élevés.
Étudier les cinétiques de réaction et le transport de masse pour concevoir des procédés pour l'industrie de la chimie fine, comme les procédés d'extraction et de distillation.
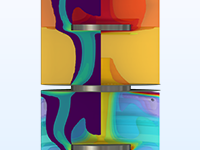
Optimiser les designs et les processus pour les applications biotechnologiques et pharmaceutiques en modélisant le transport et les réactions de médicaments dans les tissus et à travers les membranes.

Investiguer le transfert de chaleur et les réactions au cours de la pasteurisation ou étudier d'autres procédés de l'industrie alimentaire, tels que le séchage, la cuisson, et la fermentation.
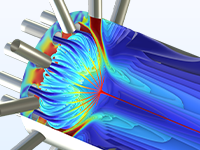
Modéliser la désactivation du catalyseur et les pertes de charge dans les réacteurs à lit fixe.

Investiguer l'élimination de polluants des effluents en utilisant des procédés de séparation tels que l'adsorption, la filtration sur membrane, et la cristallisation.
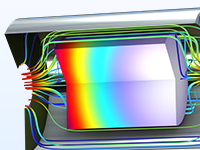
Etudier la vitesse de dépôt chimique en phase vapeur (CVD) en fonction de l'écoulement de fluide et les cinétiques de réaction dans un réacteur CVD en bateau.
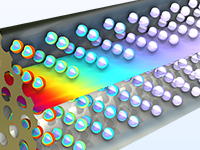
Concevoir des mélangeurs statiques pour écoulement laminaire — comme les mélangeurs pour époxy, silicones et résines acryliques — qui permettent d'obtenir les propriétés d'émulsion souhaitées.
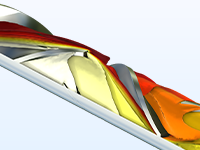
Etudier différents aspects du processus de production de polymères, comme la dissolution de réactifs, les réactions de polymérisation, le durcissement thermique, et la filtration du flux de produits.
Étudier les conditions optimales des procédés et la conception de réacteurs pour la production d'engrais, pesticides et herbicides.
Les descriptions réalistes des systèmes réactifs dans les études scientifiques et en ingénierie doivent intégrer à la fois les phénomènes de transport et les réactions chimiques pour comprendre et optimiser un procédé ou un design. Le module Chemical Reaction Engineering est adapté à la méthodologie typique des études de chimie et génie chimique, qui impliquent les étapes incrémentales suivantes :
La méthodologie de travail décrite ci-dessus peut être appliquée dans de nombreux domaines différents qui impliquent des réactions chimiques et à toutes les échelles, de la nanotechnologie et des microréacteurs aux études environnementales et à la géochimie. L'ensemble du procédé, de la définition du modèle à la présentation des résultats, est documenté dans le logiciel pour des raisons de transparence et de reproductibilité.
Le module Chemical Reaction Engineering offre une méthodologie de travail intégrée pour simuler des systèmes parfaitement mélangés en 0D, puis des phénomènes de transport en 2D et 3D.
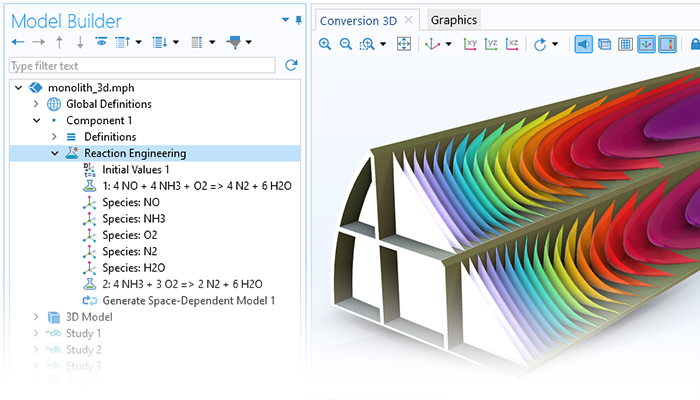
La première étape de la modélisation d'un système consiste à établir les bilans de matière. L'interface Génie réactionnel, permet d'entrer des équations chimiques et d'obtenir automatiquement les équations du bilan matière pour les espèces chimiques et les équations du bilan énergétique du système. Lorsqu'un mécanisme de réaction est saisi, les expressions cinétiques en fonction des concentrations des espèces sont dérivées automatiquement de la loi d'action de masse pour les étapes élémentaires. Les utilisateurs peuvent également saisir leurs propres expressions analytiques pour la vitesse de réaction en fonction de la concentration des espèces et de la température.
Les bilans de matière et les expressions cinétiques des réactions donnent les équations différentielles ordinaires qui sont formulées automatiquement par le logiciel. Pour un réacteur batch parfaitement mélangé, la solution des équations donne la composition du mélange réactionnel en fonction du temps.
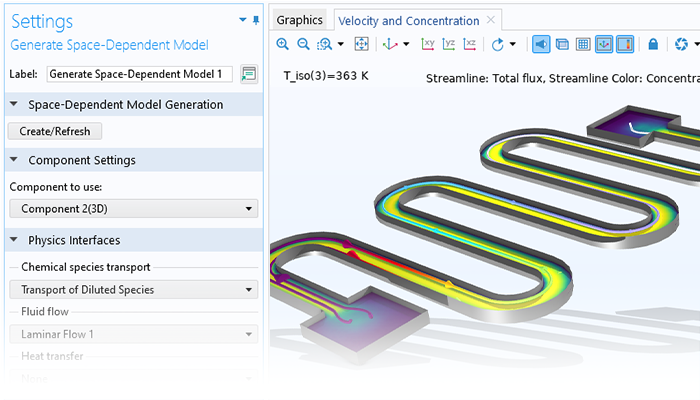
Un modèle fonctionnel pour un système parfaitement mélangé peut être utilisé pour définir automatiquement les bilans de matière, d'énergie et de quantité de mouvement pour les systèmes dépendant de l'espace. Les propriétés de transport calculées dans l'interface Génie réactionnel (par exemple, la capacité thermique, la conductivité thermique, la viscosité et la diffusivité binaire) sont automatiquement transférées aux interfaces physiques pour le transport des espèces chimiques, le transfert de chaleur et l'écoulement des fluides. Cette fonctionnalité permet d'affiner et de perfectionner les expressions cinétiques et thermodynamiques des réactions chimiques avant de passer aux modèles 2D, 2D axisymétriques et 3D.
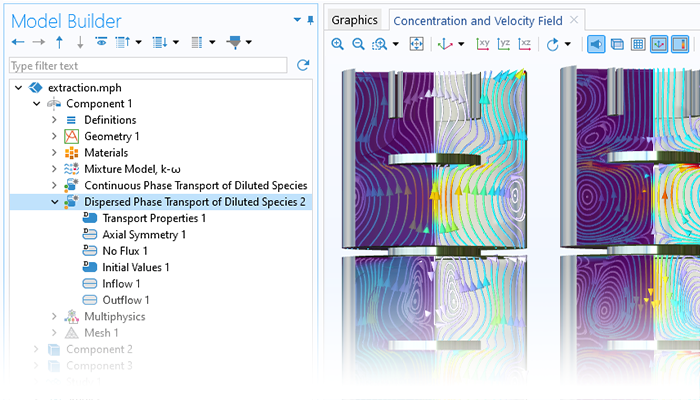
La modélisation des phénomènes de transport dans les systèmes en réaction implique la description des espèces chimiques dans des modèles dits de transport multicomposants. Le module Chemical Reaction Engineering contient des modèles sophistiqués de transport multicomposants dans l'interface Transport d'espèces concentrées, qui propose la formulation de Maxwell-Stefan et les modèles de transport multicomposants à moyenne de mélange. Pour les solutions diluées, l'interface Transport d'espèces diluées, qui traite les cas où les interactions dans la solution sont dominées par les interactions soluté-solvant, est disponible. L'interface Ecoulement diphasique dispersé avec transport d'espèces peut être utilisée pour décrire le transfert d'espèces chimiques entre deux phases fluides non miscibles. Les équations de transport des espèces chimiques sont également disponibles pour les milieux poreux, par exemple pour inclure la diffusion de Knudsen. Le modèle de diffusion Dusty Gas est également inclus. La formulation du modèle de bilan massique ainsi que les propriétés de transport peuvent être obtenues directement à partir des équations chimiques lors de la génération d'un modèle dépendant de l'espace à partir de l'interface Génie réactionnel.
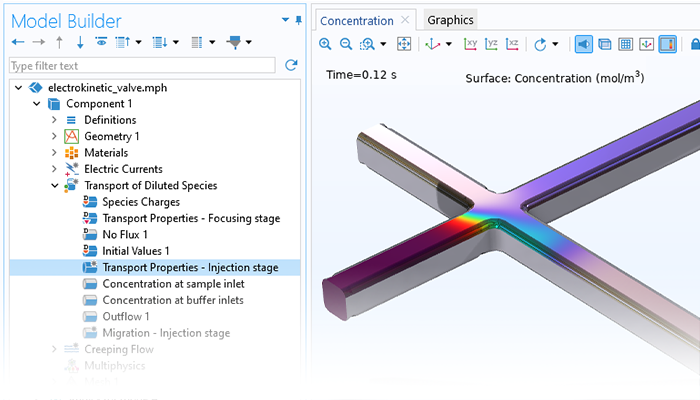
Lorsque l'on modélise le transport d'espèces diluées ou concentrées, des champs électriques peuvent être inclus comme forces motrices du transport pour la modélisation des électrolytes et des ions. Les interfaces Nernst-Planck et Transport électrophorétique sont dédiées à la modélisation des électrolytes et peuvent inclure les formulations de l'équation de Poisson ou la condition d'électroneutralité pour l'équilibre des charges dans l'électrolyte. Les applications de cette fonctionnalité comprennent les vannes électrocinétiques, les écoulements électro-osmotiques et l'électrophorèse.
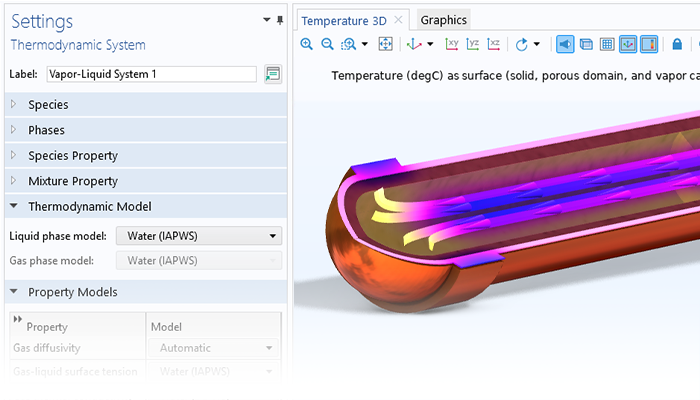
Le module Chemical Reaction Engineering contient une base de données de propriétés thermodynamiques, utilisable pour calculer les propriétés des mélanges de gaz, des mélanges de liquides, des systèmes gaz–liquide à l'équilibre (calculs flash), des systèmes liquide–liquide, et des systèmes gaz–liquide–liquide à l'équilibre. Il existe une variété de modèles thermodynamiques qui peuvent être utilisés pour calculer la densité, la capacité thermique, l'enthalpie de formation, l'enthalpie de réaction, la viscosité, la conductivité thermique, la diffusivité binaire, l'activité et la fugacité. Pour en savoir plus sur cette fonctionnalité, consulter la page du module Liquid & Gas Properties, qui est incluse intégralement dans le module Chemical Reaction Engineering.
La base de données de propriétés thermodynamiques peut être utilisée pour créer un ensemble de propriétés pour un système de réactions spécifiques en sélectionnant les espèces chimiques présentes dans le système, les propriétés souhaitées et le modèle thermodynamique. Lors de la définition des mécanismes de réaction, les réactifs et les produits peuvent être mis en correspondance avec les espèces chimiques dans le package des propriétés défini par la base de données des propriétés thermodynamiques. Cette correspondance lie automatiquement les fonctions et les équations générées par le package de propriétés au modèle du système réactionnel.
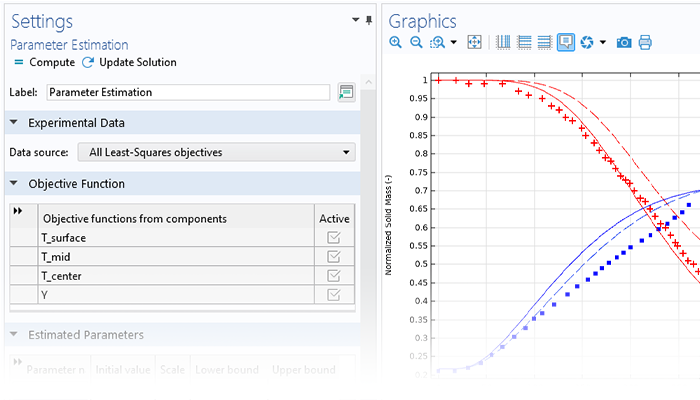
Les études des réactions chimiques et des mécanismes réactionnels reposent généralement sur l'estimation des facteurs de fréquence, des énergies d'activation et d'autres paramètres susceptibles de décrire quantitativement les observations expérimentales. Le module Chemical Reaction Engineering comprend des fonctions dédiées à l'estimation de paramètres, conçues pour optimiser ces paramètres à l'aide de données issues de multiples expériences.
La méthodologie de travail typique pour réaliser une estimation des paramètres d'un modèle est la suivante: D'abord, les paramètres du modèle à estimer (tels que les constantes de vitesse) sont sélectionnés, et les valeurs initiales et les échelles pour ces paramètres sont définies. Ensuite, les données expérimentales sont importées et les colonnes de données sont corrélées avec les variables du modèle en saisissant les noms des variables ou les expressions appropriées.
Puis, une méthode d'optimisation est choisie et calculée. La progression de l'optimisation peut être suivie pendant le calcul. Une fois que les valeurs optimales des paramètres ont été trouvées, les résultats peuvent être comparés aux mesures expérimentales.
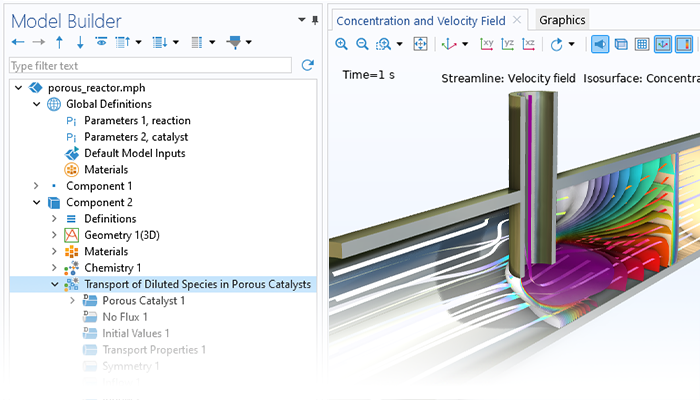
La fonctionnalité d'écoulements de fluides incluse dans le module Chemical Reaction Engineering traite les écoulements laminaires et en milieu poreux. De plus, lorsqu'il est combiné avec le module CFD, il existe des couplages prêts à l'emploi pour la modélisation du transfert d'espèces chimiques dans un écoulement turbulent. La formulation du modèle d'écoulement du fluide ainsi que la viscosité et la densité peuvent être obtenues directement à partir des équations chimiques lors de la génération d'un modèle dépendant de l'espace à partir de l'interface Génie réactionnel.
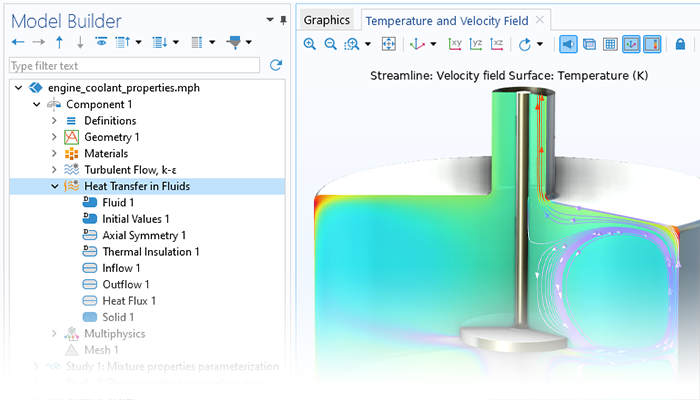
La fonctionnalité de transfert de chaleur incluse dans le module Chemical Reaction Engineering prend en compte le transfert de chaleur par conduction, convection et rayonnement. Le terme de rayonnement est donné par le rayonnement de la surface vers l'environnement, tandis que le module Heat Transfer est nécessaire pour le rayonnement de surface à surface et le rayonnement dans les milieux participatifs. Les capacités de transfert de chaleur du module Chemical Reaction Engineering comprennent le transfert de chaleur dans les fluides, les solides et les milieux poreux. La formulation du modèle de transfert de chaleur, ainsi que les propriétés thermodynamiques et de transport, peuvent être obtenues directement à partir des équations chimiques lors de la génération d'un modèle dépendant de l'espace à partir de l'interface Génie réactionnel.
Les réactions en surface sont typiques de la catalyse hétérogène ainsi que des procédés de dépôt en surface comme le dépôt chimique en phase vapeur. On les retrouve dans l'industrie des produits chimiques en vrac, par exemple dans le procédé Haber-Bosch pour la production d'ammoniac et dans les microcapteurs pour la détection de très faibles quantités de traceurs qui peuvent s'adsorber sur des surfaces et être détectés, par exemple, par un changement des propriétés électriques.
Dans les modèles de transport-réaction, les réactions de surface peuvent être traitées comme des équations aux frontières couplées aux conditions limites pour les équations de transport et de réaction dans le volume. Ceci est typique pour les modèles à l'échelle microscopique ou inférieure. Alternativement, dans les milieux poreux, ces réactions sont traitées de la même manière que les réactions homogènes mais incluent la surface spécifique (surface par unité de volume du matériau poreux) et les propriétés de transport effectives. Cela serait typique des modèles à la fois à l'échelle microscopique et à l'échelle macroscopique, appelés modèles multi-échelles.
Le module Chemical Reaction Engineering comprend des formulations prêtes à l'emploi pour la catalyse hétérogène dans deux configurations : réactions de surface sur les faces de frontières ainsi que réactions de surface distribuées sur un catalyseur poreux homogénéisé. Pour les catalyseurs poreux, des modèles multi-échelles sont prédéfinis pour décrire des structures poreuses bimodales. Ces structures peuvent être constituées de granulés microporeux compactés pour former un lit de granulés macroporeux.
Chaque activité et chaque besoin en matière de simulation est unique.
Afin d'évaluer pleinement si le logiciel COMSOL Multiphysics® répond ou non à vos exigences, nous vous invitons à nous contacter. En parlant à l'un de nos représentants, vous obtiendrez des recommandations personnalisées et des ressources détaillées qui vous aideront à tirer le meilleur parti de votre évaluation et vous guideront pour choisir l'option la plus adaptée à vos besoins en matière de licence.
Il vous suffit de cliquer sur le bouton "Contacter COMSOL", d'indiquer vos coordonnées et tout commentaire ou toute question spécifique et de soumettre votre demande. Vous recevrez une réponse d'un représentant de COMSOL très rapidement.
Demander une démonstration du logiciel