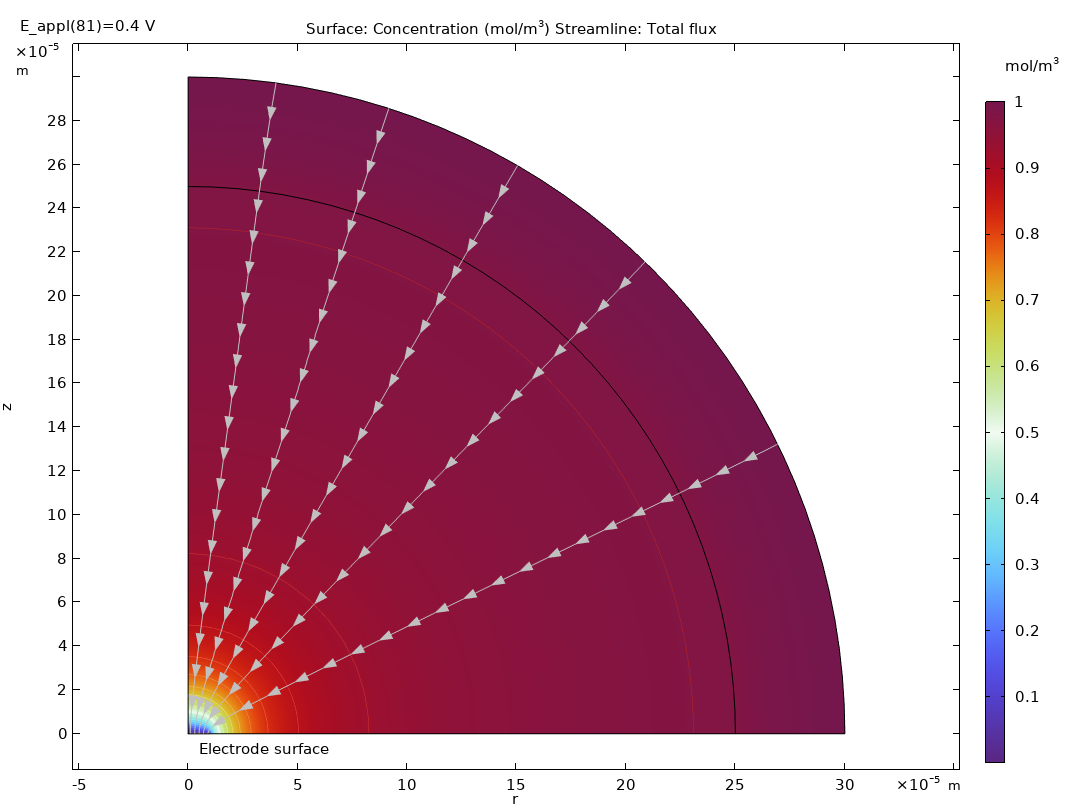
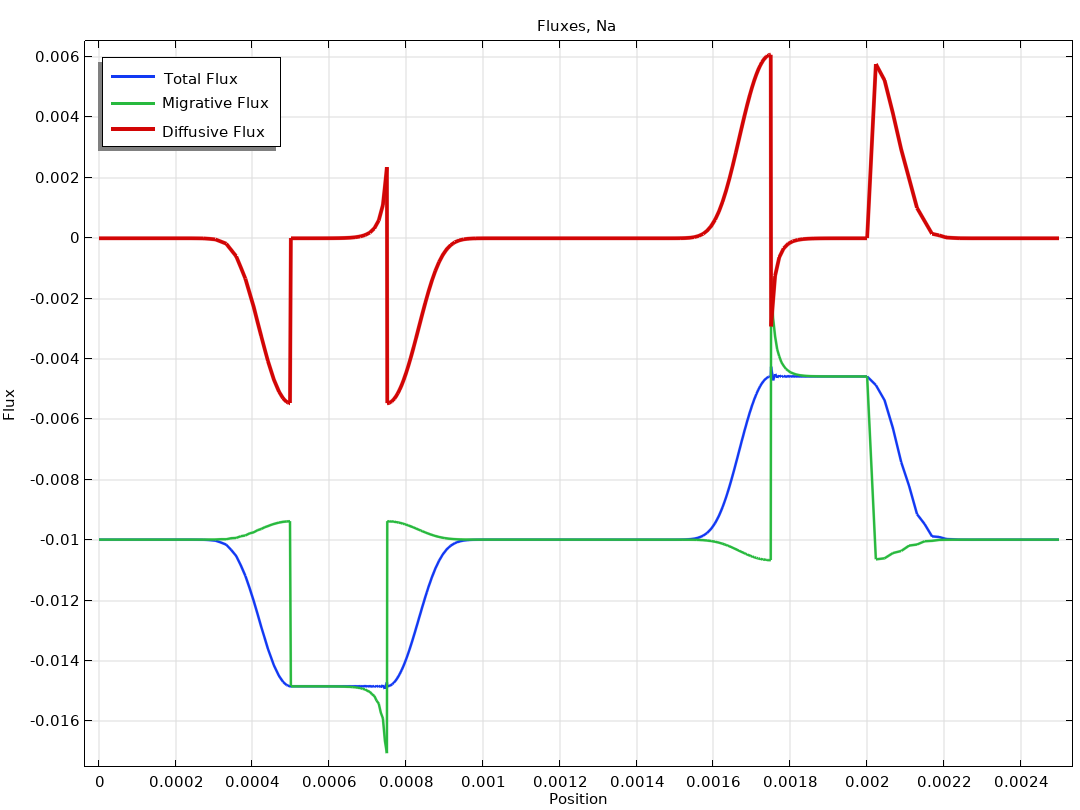
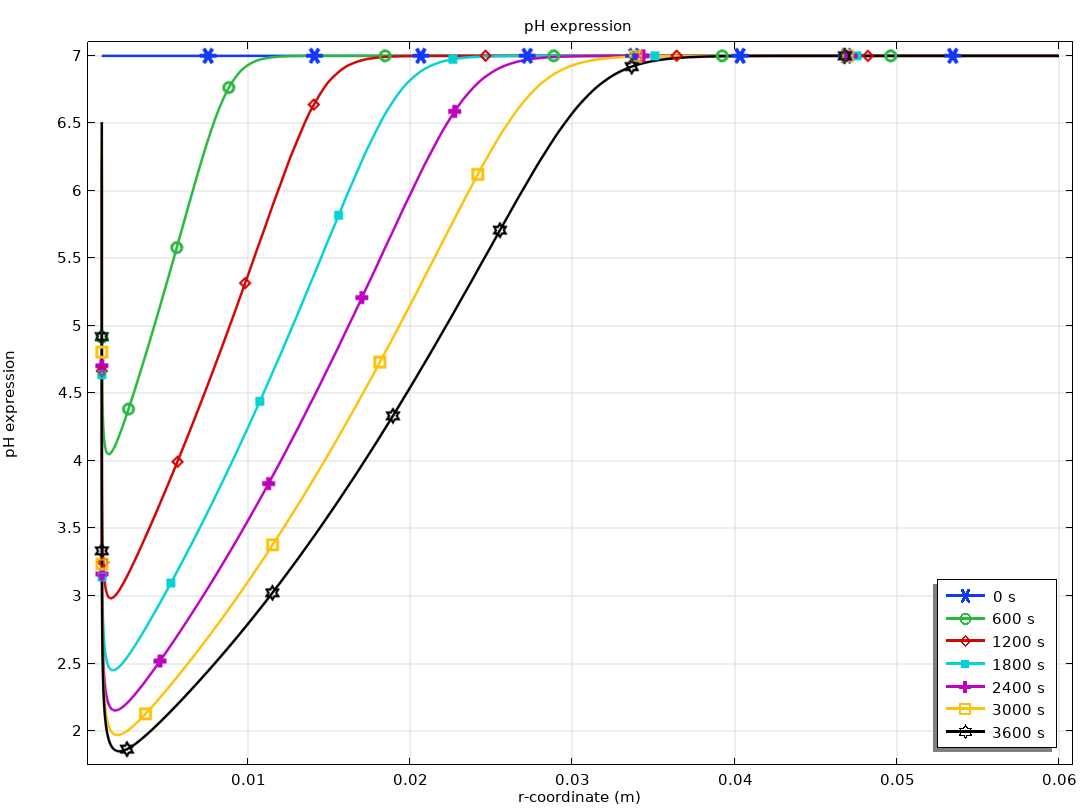
Méthodes électroanalytiques
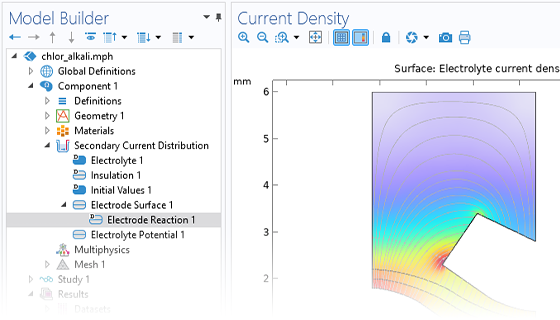
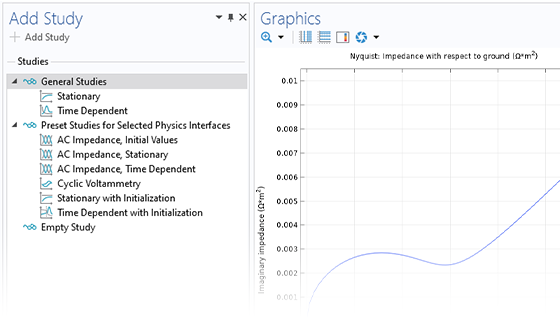
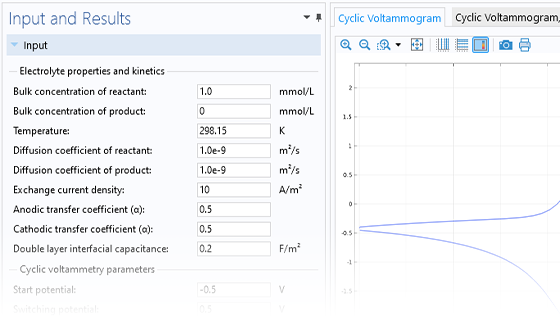
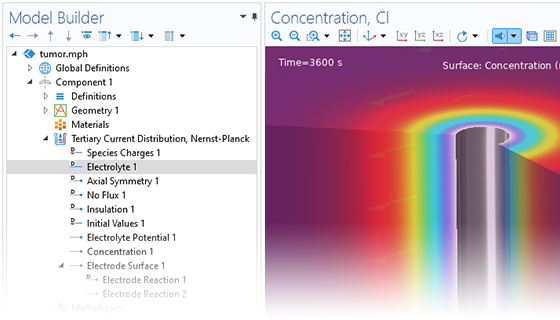
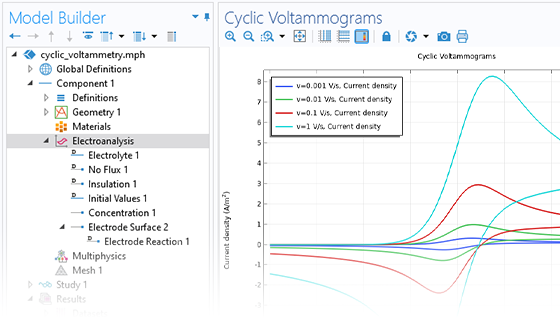
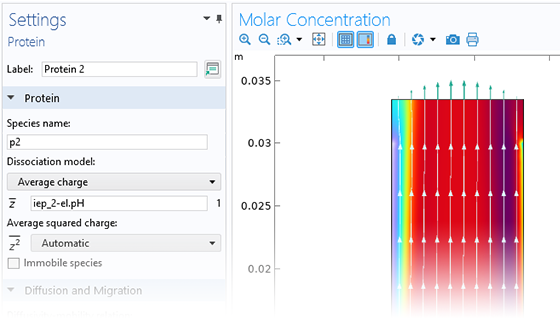
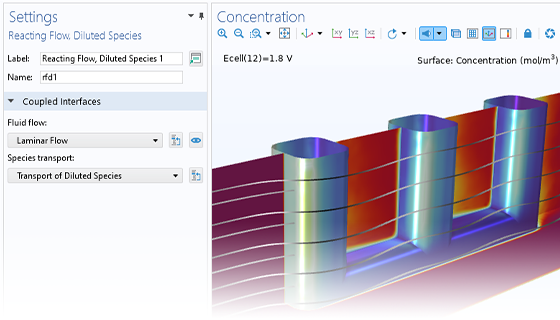
Le module Electrochemistry permet de simuler les méthodes électroanalytiques les plus courantes, comme la voltampérométrie (cyclique), l'ampérométrie et la chronoampérométrie, la potentiométrie, la coulométrie et la spectroscopie d'impédance électrochimique (EIS). Ces méthodes peuvent être utilisées pour la modélisation et l'analyse d'une solution électrolytique statique ou soumise à un écoulement de fluide forcé. En associant le module Electrochemistry et le module Optimization, et en combinant des résultats expérimentaux et de simulation, les utilisateurs peuvent estimer des paramètres, tels que les densités de courant d'échange, les coefficients de transfert de charge, les surfaces actives spécifiques, les diffusivités et les mécanismes de réaction. Ces propriétés peuvent ensuite être utilisées dans des applications industrielles à des fins de modélisation et d'optimisation de design.