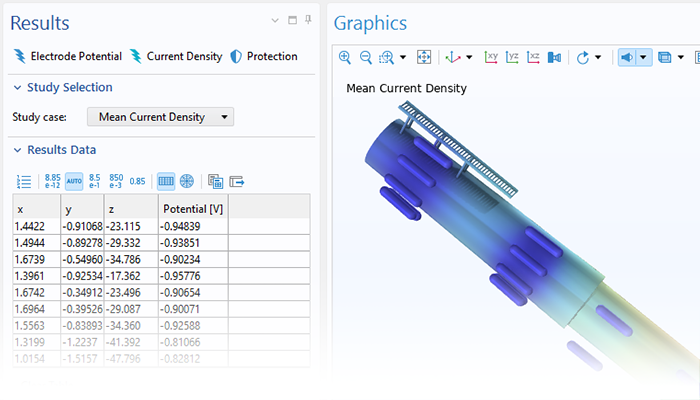
Processus de corrosion
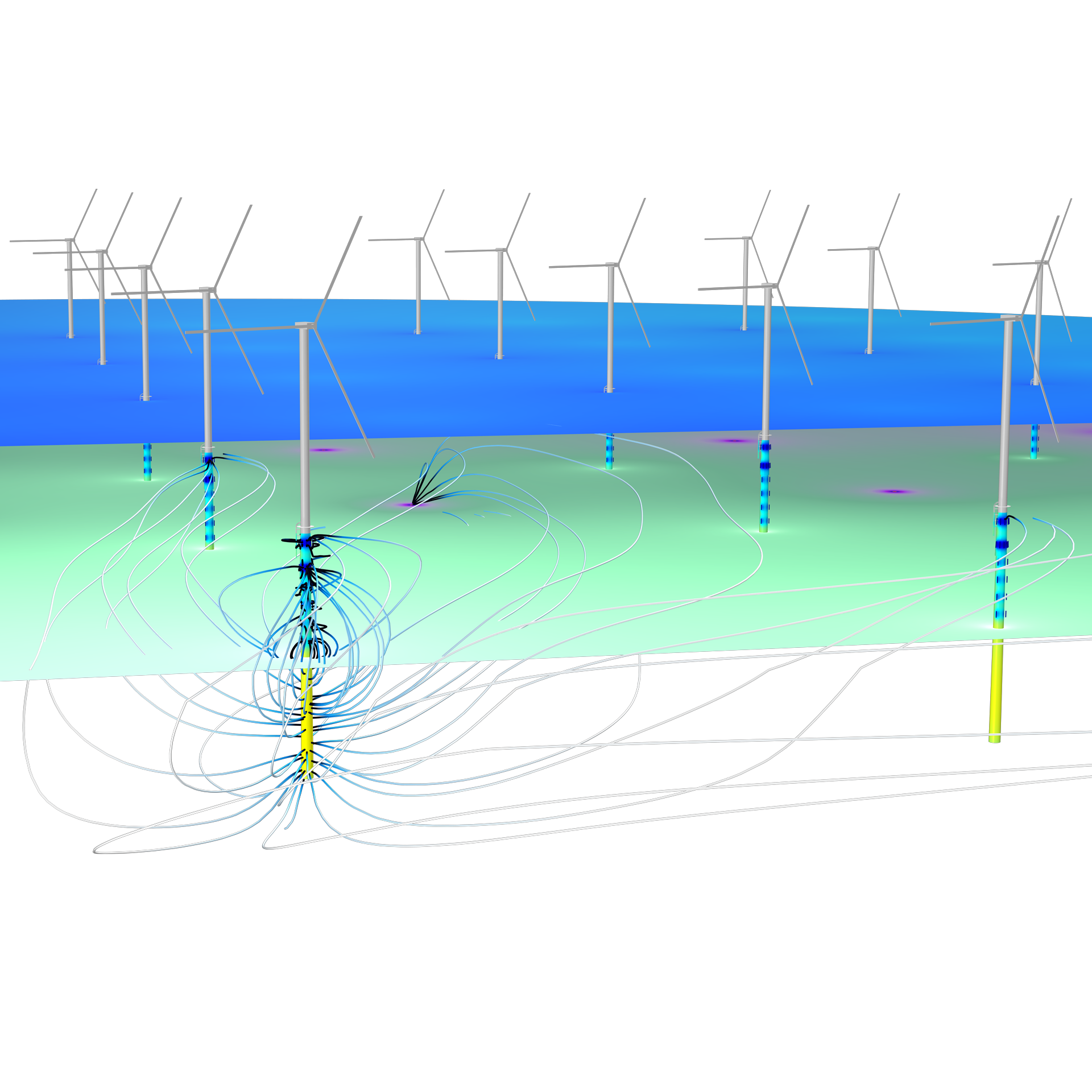
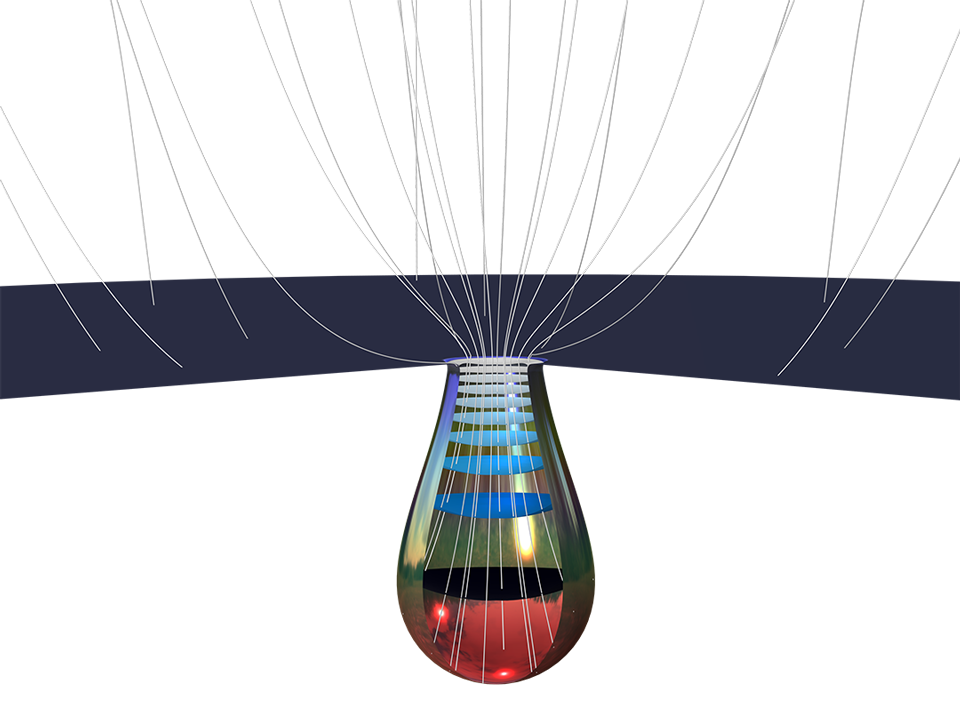
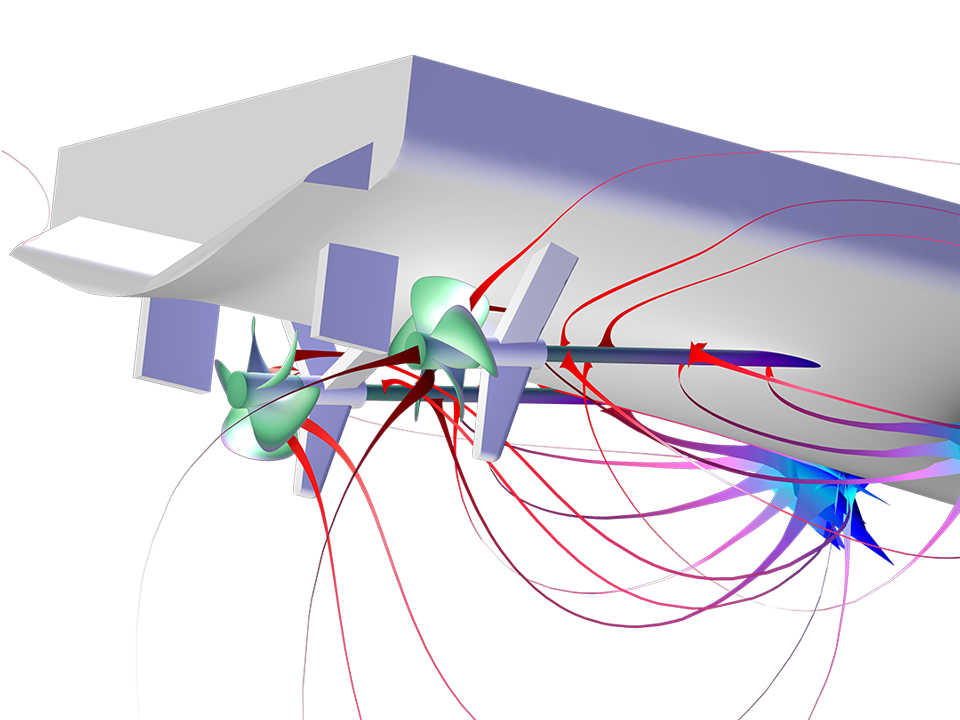
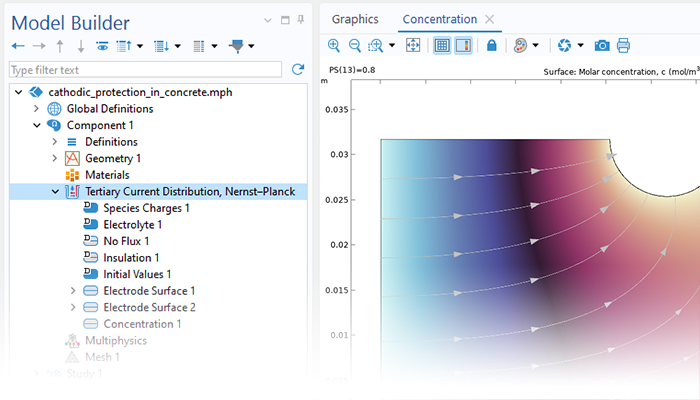
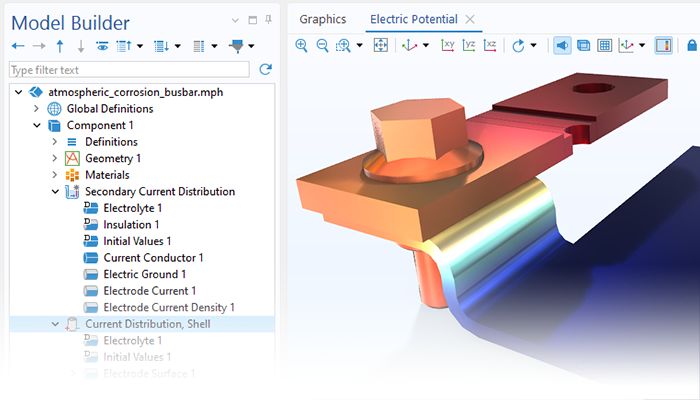
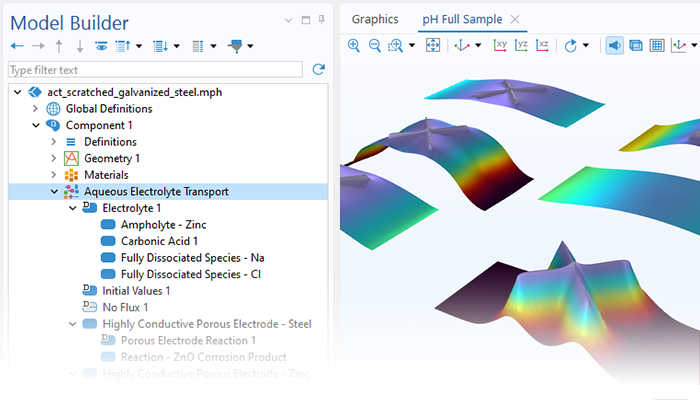
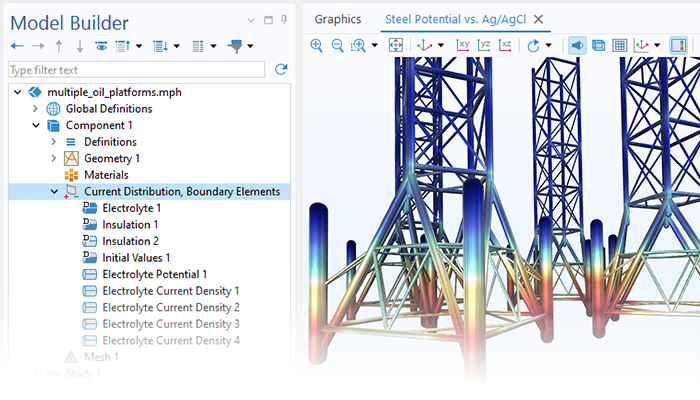
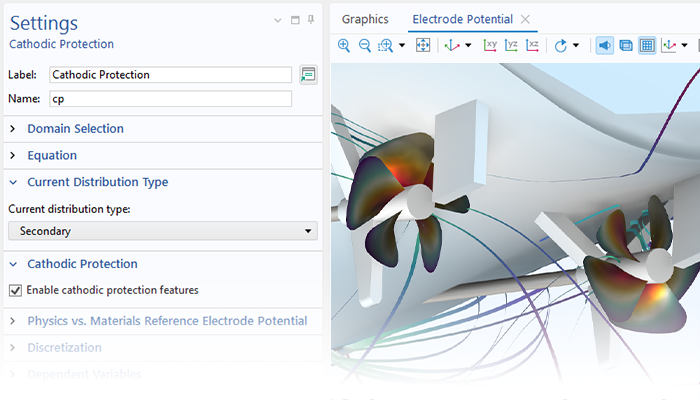
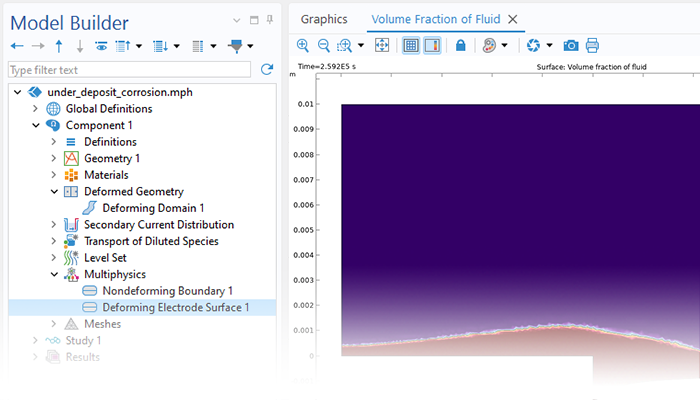
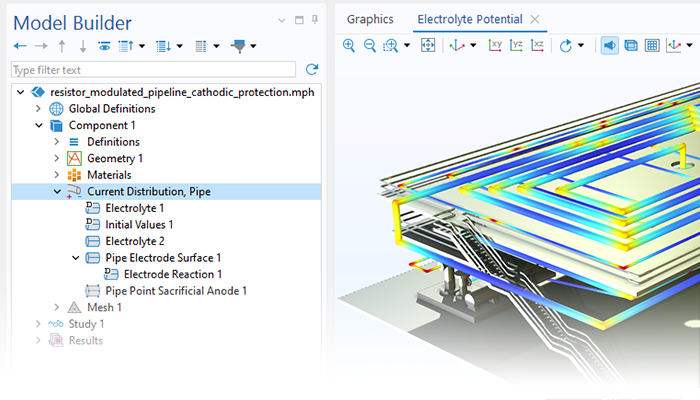
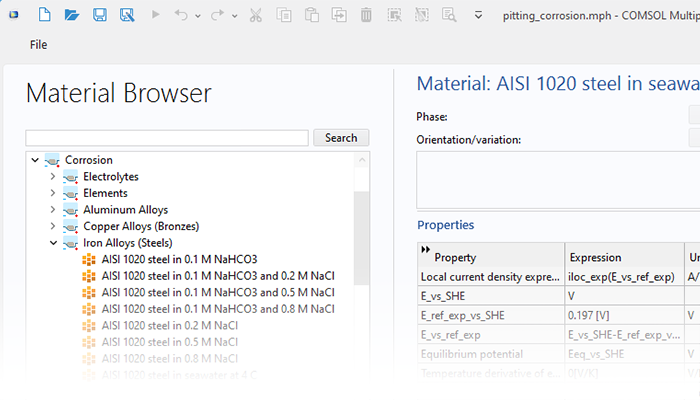
Le module Corrosion est utilisé pour modéliser une variété importante de phénomènes de corrosion : galvanique, atmosphérique, par piqûres, par crevasses, et corrosion générale. Il est également utilisé pour modéliser la corrosion dans les systèmes pétroliers et gaziers, dans le béton armé et la corrosion due aux courants vagabonds.
Ces processus de corrosion découlent de phénomènes électrochimiques de types similaires pour lesquels le transport et l'équilibre de la charge électrique et de la masse doivent être considérés. Le module permet aisément de définir les conditions aux limites, les réactions de surface et les conditions de l'électrolyte de masse. L'électrolyte peut être décrit comme une fine couche d'humidité, un matériau poreux ou un électrolyte liquide.
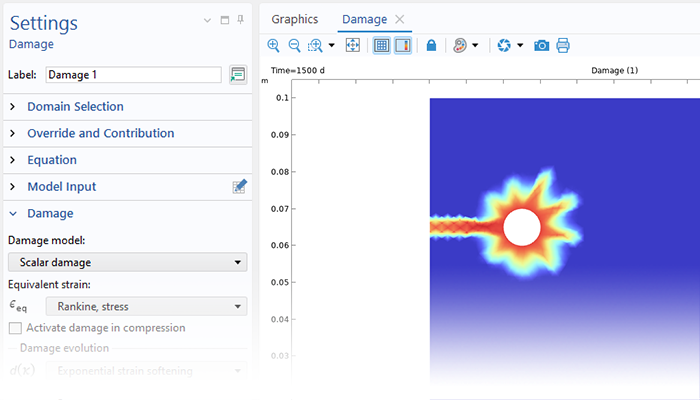
Grâce aux capacités intrinsèques de la plate-forme COMSOL Multiphysics®, le module Corrosion est combinable avec d'autres modules complémentaires pour modéliser des phénomènes couplés, comme les transferts thermiques et la mécanique des structures. Cette modélisation multiphysique est possible pour un certain nombre de phénomènes intervenant dans des problèmes de type corrosion sous contrainte (SCC) et dilatation par la rouille dans les bétons armés.